Pioneering Research in MS
A Phase I Study of Autologous Bone Marrow-Derived Mononuclear Stem Cells (BM-MNC) and Liberation Therapy (When Associated With CCSVI) in Patients With RRMS (2010 – 2016)
- Breakthrough therapy for the treatment of Multiple Sclerosis
- Site-specific (cerebral) delivery protocol with a demonstrated clinical proof of efficacy
- One treatment with ameliorative and often ‘curative’ results
- Technology transferable to other neurodegenerative diseases (e.g. Parkinson’s, ALS, dementia, CTE)
- Current reimbursement revenue stream over $1mil
Addressing Unmet Needs in the Treatment of MS
- There is little evidence that disease modifying therapies, such as Tecfidera, are beneficial in reversing progressive (non-remitting) MS
- Current therapies do not prevent inevitable disability
- There are no treatments that restore function
- Current drug regimens have numerous and serious adverse effects and deaths have occurred
Multiple Sclerosis as a Vascular Disease
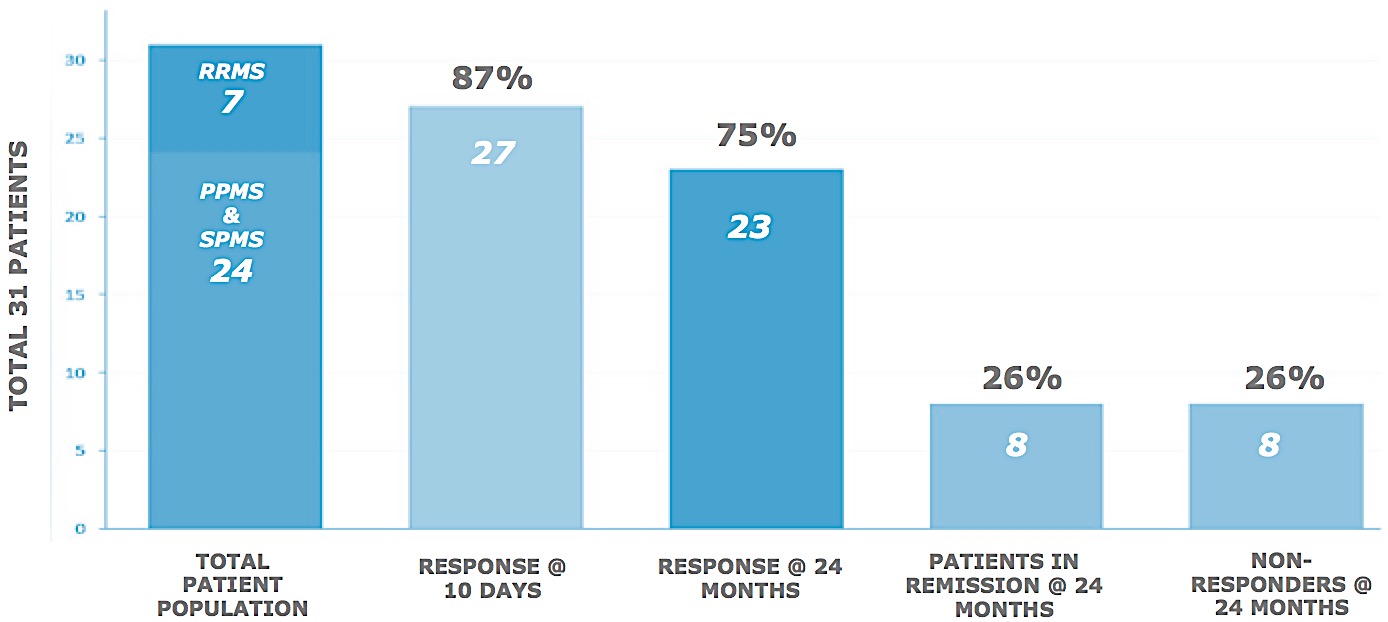
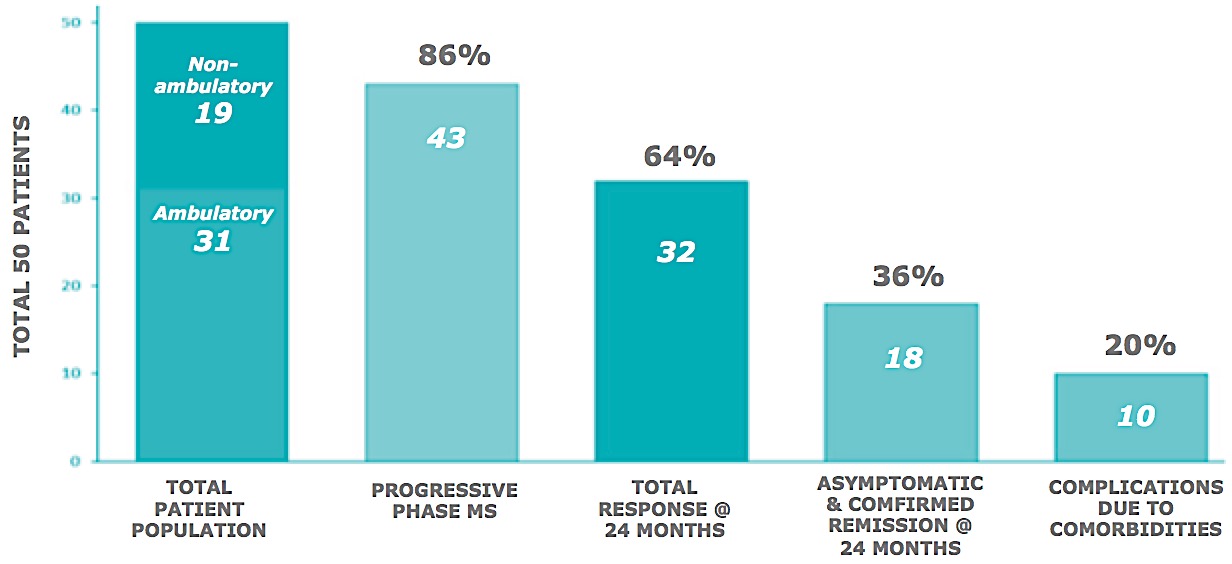
In 50 MS patients, many with the most debilitating manifestations of the disease, response to treatment was measured by functional improvement. Amongst the ambulatory group, there was a 22% improvement in function at 10 days in 90% of the patients. In most cases, subjects continued to improve over time and at two years, 25% of the study population are symptom free with many of the remaining patients having significantly reduced need for medications.
Improvements observed through Brain Imaging
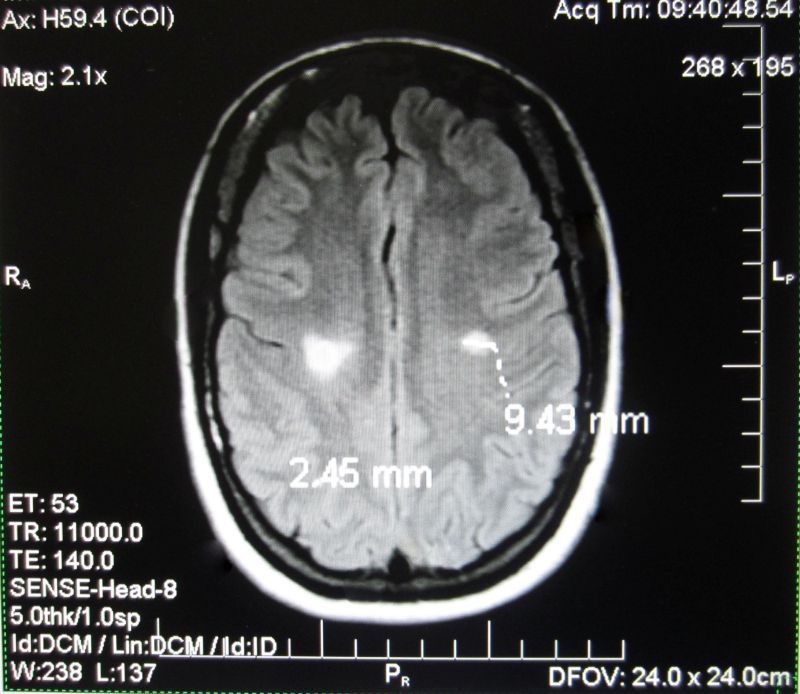
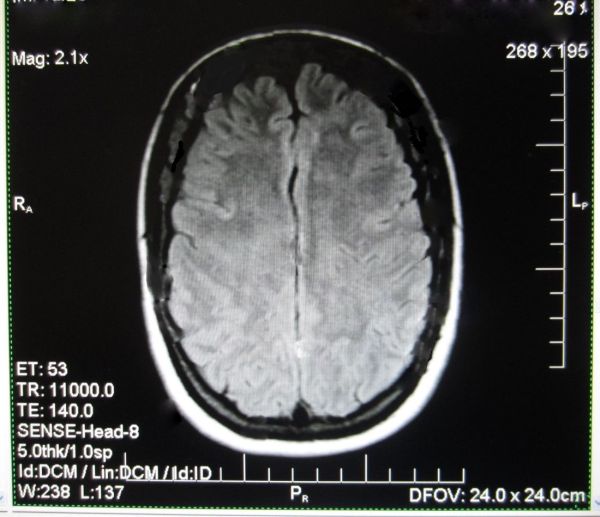
This patient was treated Sept 2013: same sequence of scans from Patient, 6 months apart. T2 hyperintense white matter lesions regularly disappear as the brain repairs itself in the relapsing remitting phase of MS. Mostly the lesions reoccur within months and the myelin does not fully repair as symptoms worsen.
However this patient did not experience a reoccurrence of lesions as her symptoms improved and she went back to work as a government translator (see next image). Her neurologist subsequently confirmed her remission, which is part of her medical record.
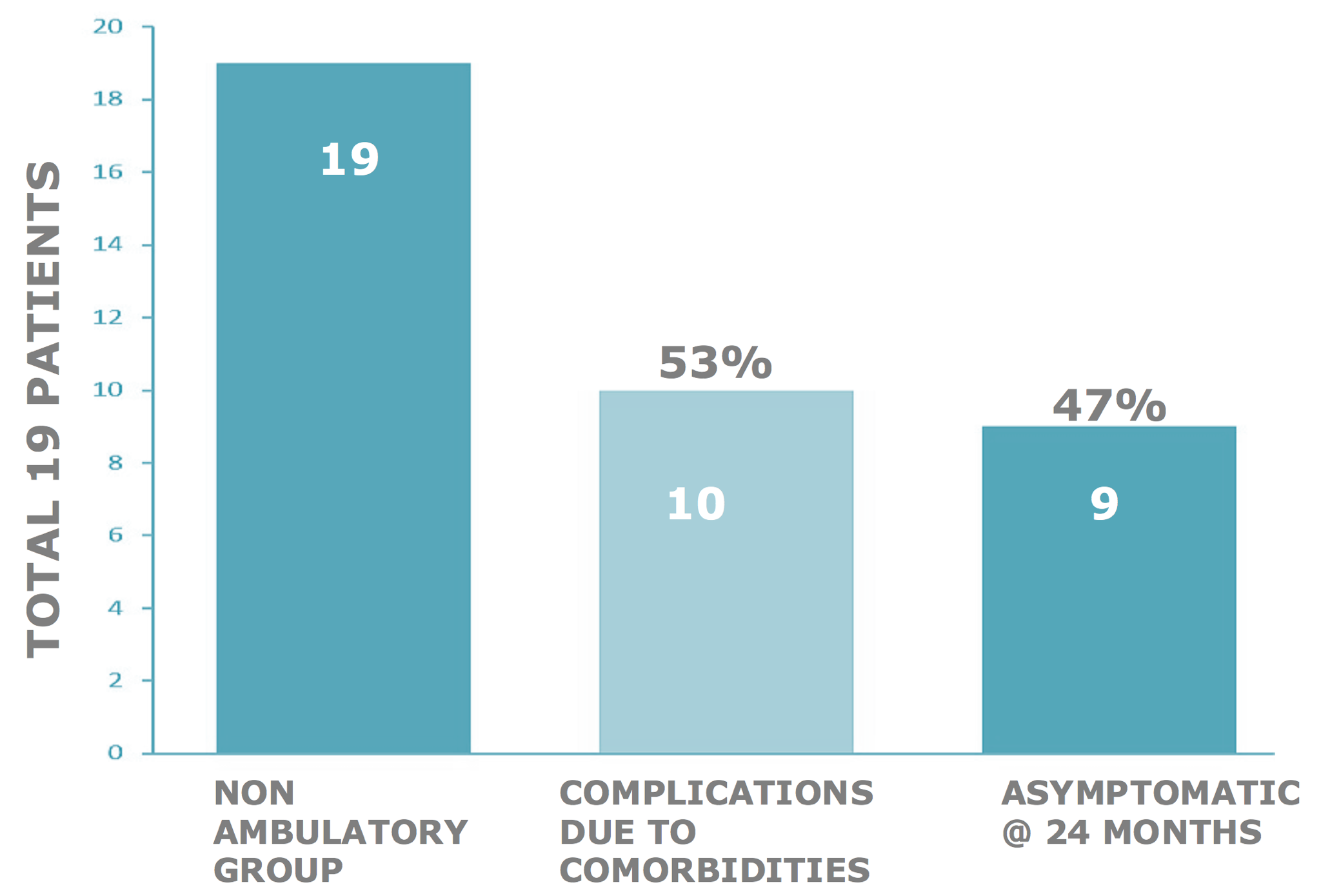
Non-ambulatory study subject response rate @ 24 months
Confounding evidence
We uniquely discovered that MS patients who had been non-ambulatory with MS for many years developed osteoporosis, joint contractures, nerve root compression – radiculopathy, or spinal stenosis – myelopathy, which can mimic some symptoms of MS. Recovery of function must address these aspects with the physiotherapy / surgery strategies in use for these conditions. Study subjects who took active and regular physiotherapy, post-therapy recovered more function than those who did not.
Somata SupraselectiveTM
Competitive Advantages
- Potential for a “Breakthrough Therapy Designation”under Section 902 of FDA’s Safety and Innovation Act
- Competition has limited efficacy and not approved for the most severe forms of MS
- Supraselective stem cell therapy has immediate functional improvements
- and, at two years >25% of patients had complete remission
- Supraselective stem cell therapy is a one-time treatment
- Supraselective therapy is safe; no observed adverse effects at 2 years
Symptoms are reduced or eliminated with less need for medications, even in patients for whom other therapies have not been effective. Restored function over time has been demonstrated.
Multiple Sclerosis
Health Economics
- Cost of Tecfidera, current drug of choice, is $78k/yr.
- Co-medications to treat sequelae of MS approximately $60k/yr.
- Assistive devices and physiotherapy in advanced phases $30k/yr.
- Life supportive devices and assisted living in acute phase $100k/yr.
- HEALTH ECONOMIC BURDEN: ~ $2.5mil over 10 years.
- Somata Supraselective is a one-time treatment protocol with a post-registration proposed price of $150k (US).
Common MS complications due to spinal stenosis
Myelopathy in the non-ambulatory group (from actual study subjects)
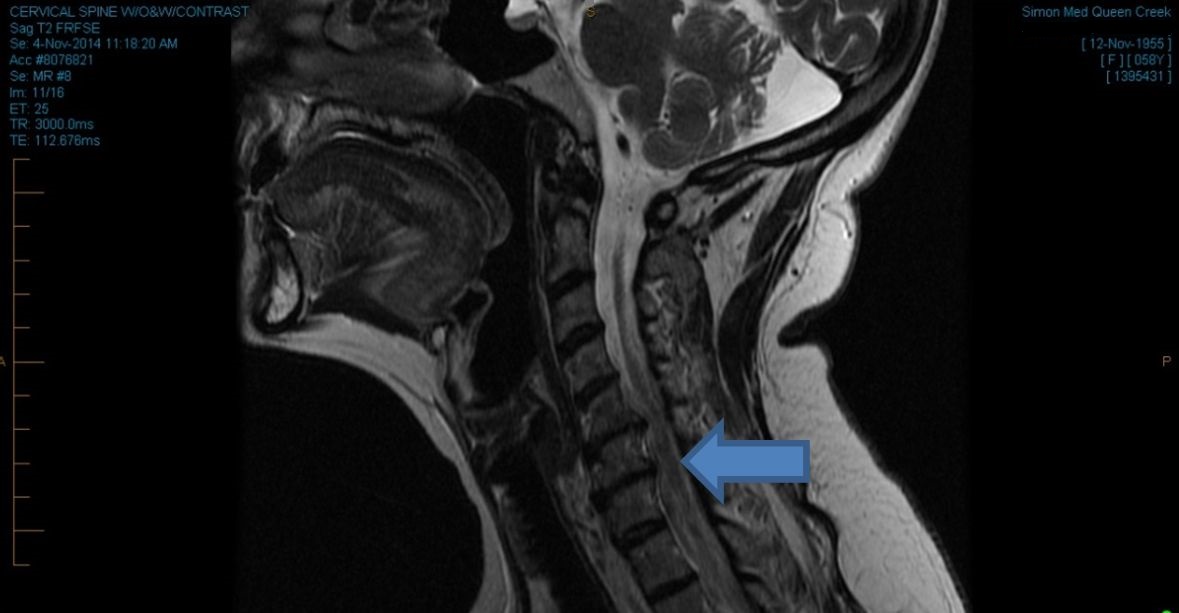
Patient 1. A rectification of the normal cervical lordosis can be seen throughout spinal levels C5-C7. Discprotrusions are evident at spinal levels C5-C7 (arrow). The spinal cord is significantly compressed (arrow).
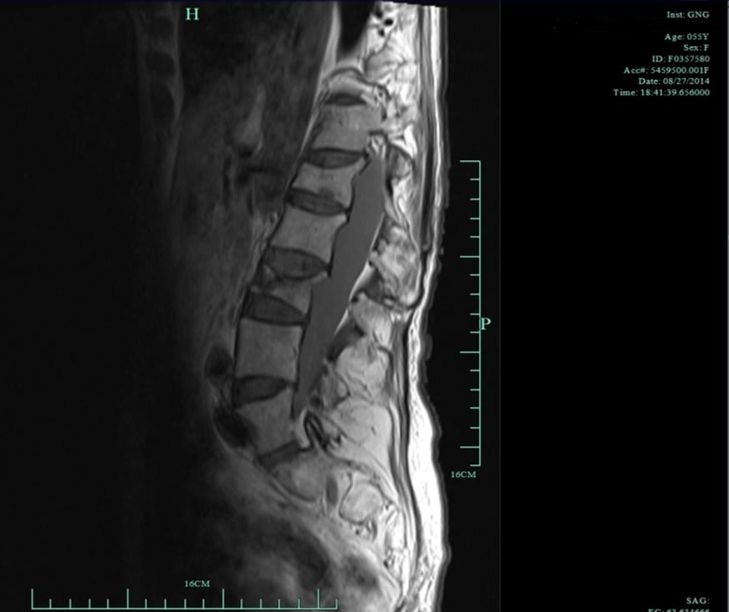
Patient 2. T2 proton density weighted sagittal image demonstrating scoliosis. The arrow points to a collapse of the anterior vertebral body at spinal cord level L3.
Comparison to Current Disease Modifying Therapies
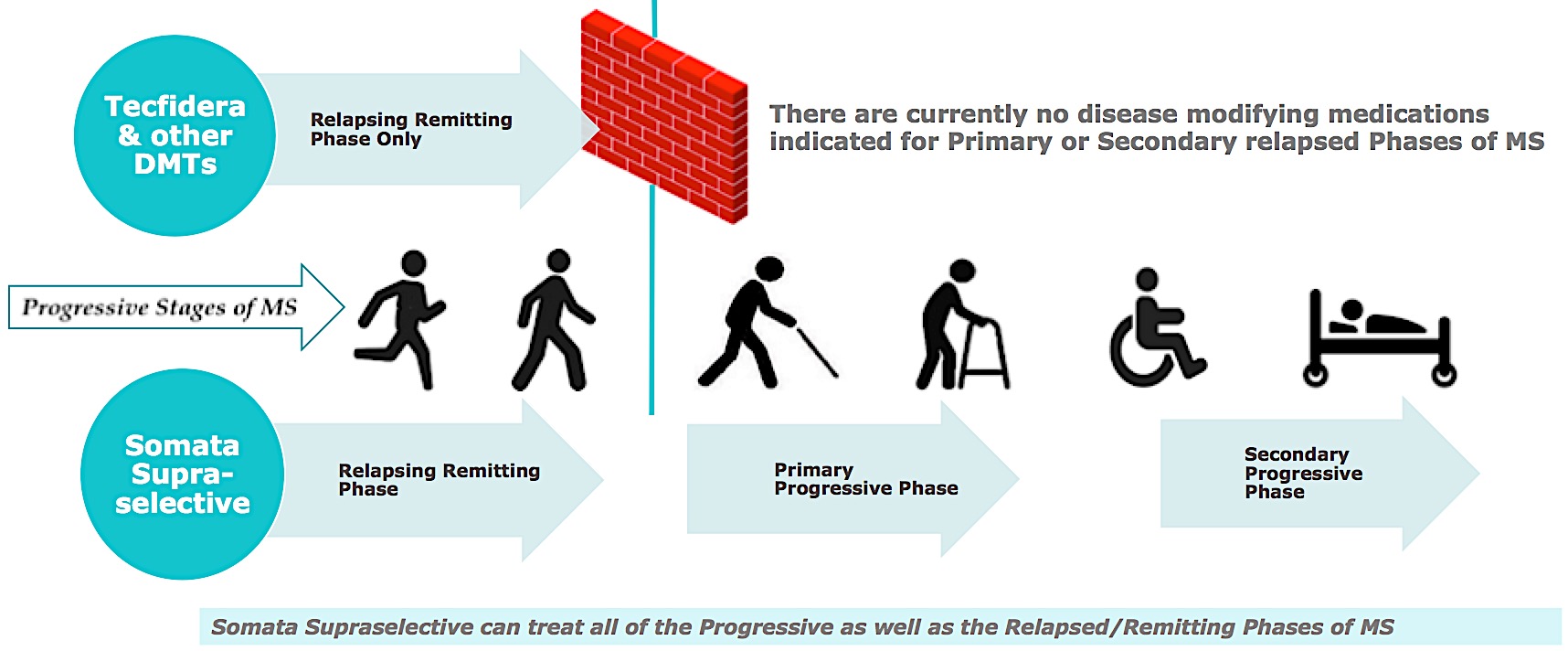
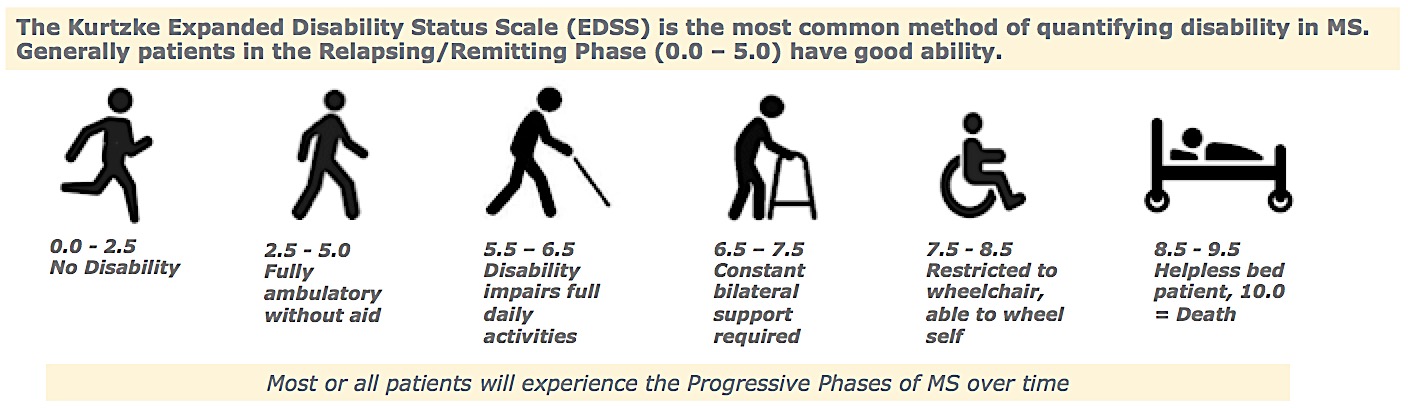
Progressive Phases of MS: Kurtzke EDSS Disability Scale
Comparison
DMT* clinical studies to Somata Supraselective trial
Tecfidera® (dimethyl fumarate) is a prescription medicine used to treat people with relapsing forms of multiple sclerosis.
Key Inclusion Criteria of Tecfidera® clinical trials were:
- Must have confirmed diagnosis of Relapsing/Remitting MS according to McDonald criteria
- Must have relapsing-remitting disease course (early phase MS), cannot have any secondary phases of MS.
- Must have a baseline EDSS between 0.0 and 5.0, inclusive.
The Future of Multiple Sclerosis Treatment
- Somata Supraselective is single therapy vs chronic pharmaco-therapy
- Clinical efficacy and safety superior to any other current therapies
- 10 yr. health economic burden a fraction of current interventions
- Projected 30% US market penetration at 5 yrs. post-registration
- Potential Sales of > $5 bil @ 5 years
World-wide MS population; approximately 2.5 mil, 5 mil predicted in 2025.
Background of the invention
The invention relates to methods and compositions for treating a patient having or at risk of developing a vascular-related degenerative neurological disease(s) or disorder(s), e.g. multiple sclerosis, by administering stem cells to the patient.
Original work by Alphonse (Franz) Schelling that guided our research forward from 2010 was confirmed by multiple other scientific papers dating back to the 1850s.
“I made observations which turned my attention to Multiple Sclerosis which were widenings like varicose veins in skulls which I found in the archives of the neurological hospital in Salzburg in 1982/83. Of the skulls showing abnormal widenings of the transition between the sigmoid sinus and internal jugular vein, 80% had a diagnosis of MS. So I was forced to study the literature and I was amazed that nobody had noticed these SPECIFIC observations and specific kind of changes in the brain. THESE CHANGES WERE ALWAYS DEVELOPING AROUND VEINS NOT AROUND VENULES.”
-Franz Schelling
Vascular-related degenerative diseases afflict many people and are often neurologically based. Vascular dysfunction with respect to craniovertebral anomalies offers an explanation for symptoms of some neurological diseases for which there are no effective treatments. In the case of neuronal disorders that have a primary vascular origin, circulating neurotoxins cross the blood brain barrier (BBB) to reach neuronal targets and trigger injury (Zlokovic B. 2008). Moreover, despite medications that are prescribed only for symptoms of the effect, these diseases are often fatal. One of the effects of vascularrelated diseases results is the destruction of the myelin sheath covering neurons necessary for the proper function of the central nervous system. Multiple Sclerosis is one such vascular-related degenerative neurological disease having serious, sometimes life-threatening consequences. (Schelling F. 1986)
The Relationship
Vein & Lesion
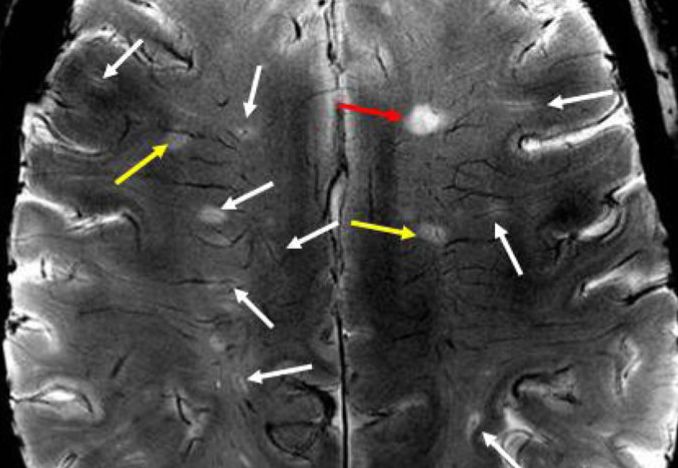
The cranial veins can only be seen on a 7-T MRI, rarely used in MS brain studies, so this connection is not well known. Lesions develop at known points of leakage around cranial veins.
Blood brain barrier leakage in MS results in the local deposition of fibrinogen, a neurotoxin. As a first step to overcoming the source of the leakage, it is essential to develop a technique to halt that occurrence.
Stem cells directed towards the cranial veins, repair the incompetent vessels, arrest aberrant leakage and the effects are immediately demonstrated by patients.
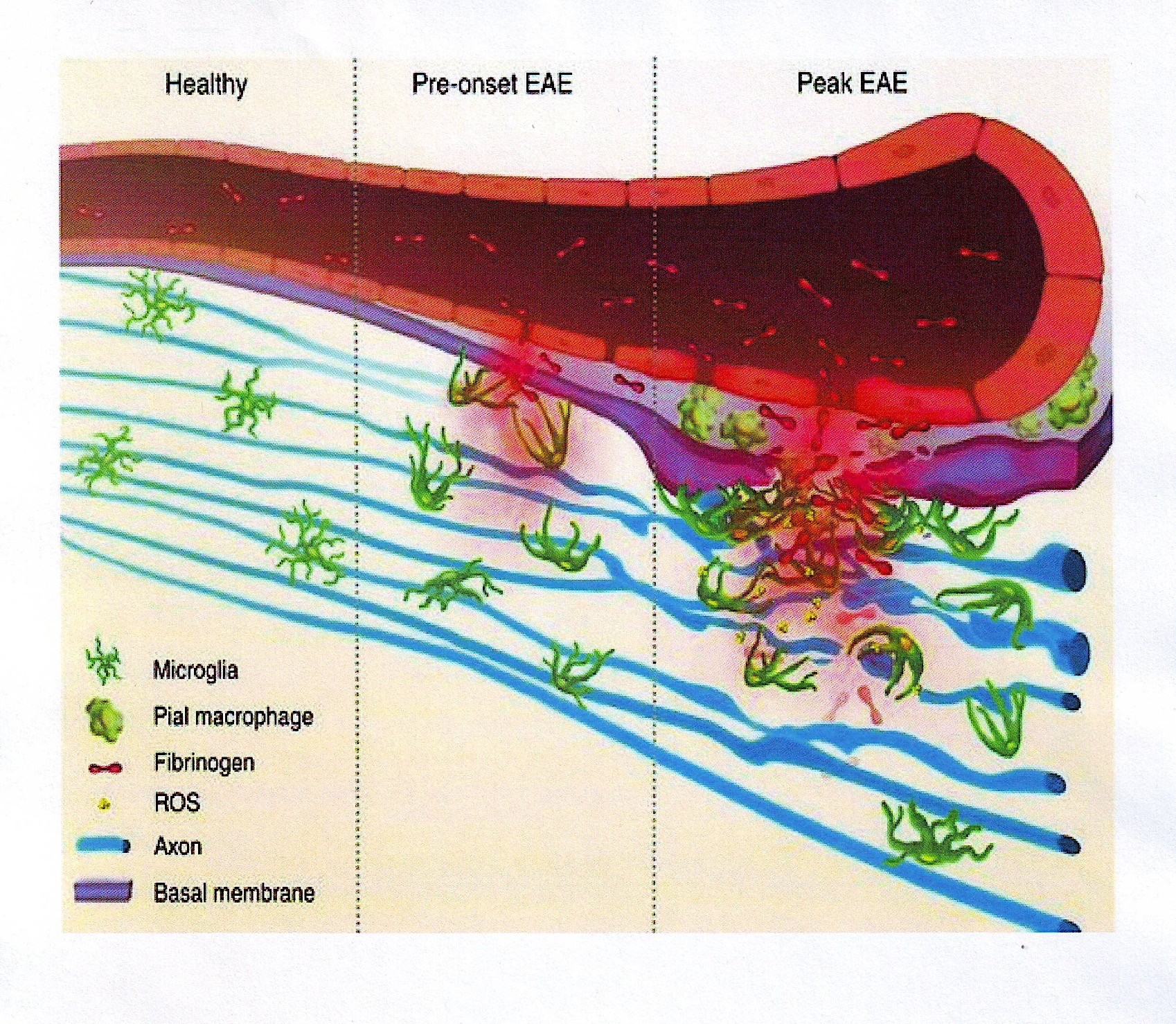
Blood-brain barrier disruption, microglial activation and neurodegeneration are hall-marks of multiple sclerosis. Recent studies show that the blood protein fibrinogen induces rapid microglial responses toward the vasculature and is required for axonal damage in neuroinflammation. Thus, early and progressive perivascular microglial clustering triggered by fibrinogen leakage upon blood-brain barrier disruption contributes to axonal damage in neuroinflammatory disease.
[*Davalos D et al (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012; 3:1227 doi: 10.1038/ncomms2230.]
Medical evidence indicates that MS begins as a brain blood vessel disorder, which leads to progressive neurological impairment (D’haeseleer M. 2011, Zlokovic B, 2008). There are an estimated 400,000 MS patients in the US alone with a worldwide population of 2.5 million. This prevalence is expected to increase greatly by the year 2025. Moreover, because patients experience progressive functional and physical decline, MS is very treatment intensive and as a consequence, very expensive to treat. In the future, MS will occupy a greater share of U.S. and world health care budgets (Hartung DM, 2015). Most patients are diagnosed between the ages of 20 and 50, and about two thirds are women. The disease is more frequently found among people raised at higher latitudes. Decreased exposure to the sun resulting in decreased vitamin D production has been suggested as a possible factor (Alharbi FM. 2015). Studies also indicate that genetic factors make certain patients susceptible to the disease, but there is no evidence that MS is directly inherited.
[https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFi les/Brochures/Brochure-What-Is-MS.pdf.]
MS is a neurodegenerative disease in which vascular abnormalities result in a cascade of events that culminates in white matter inflammation developing at locations in the brain adjacent to dysfunctional veins that have become porous and unable to contain blood proteins from crossing the blood brain barrier (Schelling F, 2014). Inflammation subsequently causes destruction of the myelin sheath (the fatty substance that coats and protects nerve fibers in the brain and spinal cord) covering neurons and axonal fibers leading to a range of signs and symptoms including improper balance, lack of normal walking ability and impaired cognition. In magnetic resonance images (MRIs), the regions involved eventually appear as white areas, or scleroses within normal-appearing brain or spinal cord tissue. Currently, MS is considered among many in the medical community to be an autoimmune disease where the immune system attacks myelin idopathically, meaning for an unknown reason, or spontaneously. (Weinshenker BG, 1994).
The bold and time-worn assertion that MS is an autoimmune disease is not without challenge in 2016, despite its presence on nearly every list of such diseases. The main criterion of any given autoimmune disease is that a precise auto-antigen must be present in all patients with the disease. Despite decades of attempts to identify various proteins, lipids, and gangliosides in myelin as potential MS antigens, none have been found or confirmed, nor does MS meet any of the other five criteria necessary for that statement. For example, investigators have attempted to show that CD4+T cells play a pathogenic role in the development of MS. Unfortunately many findings regarding the role of CD4+T cells have not been reproduced elsewhere. Even a cursory review of the literature will reveal that potential explanations of the pathophysiology of MS abounds with confusion as researchers endeavor to classify the disease as either pathological or clinical depending on their predisposition. Instead, the diagnosis of MS does not rely on either sound pathognomonic or observational
criteria, but a combination of ruling out other diseases and multiple other tests (Polman C. et al 2011). Of course the immune system plays a critical role in the development of lesions, especially during the acute early phases of the disease characterized by inflammation, often leading to relapses. Recent studies at least partially refute the autoimmune hypothesis (Wootla B, 2012). However, the hypothesis described herein that explains the immune-mediated pathogenesis of MS is by no means newly conceived; it is the oldest clinical evidence that exists as to the cause. For almost 200 years, physicians have observed that the autoimmune response in MS is secondary to the vascular disorder. Upon a review of the literature over generations, it is clear that a significant number of researchers, including Jean-Martin Charcot, the Father of Modern Neurology, have convincingly demonstrated the Vein-Lesion Connection in MS, and there is much experimental evidence to support the hypothesis (Ge Y 2008, Kumar D 2011, Gulcher JR, 1994, Chaudhuri A 2004, Schelling F 2012). The disease is characterized in its first stages by a failure of or leaking of fluid through the blood brain barrier (BBB) and subsequent infiltration and local dispersal of blood-borne inflammatory cytokines into the central nervous system (CNS) (Larochelle C et al 2011).
There are three main courses, or patterns of progression in multiple sclerosis (Poser CM. 1983). In each case, MS symptoms may be mild, moderate or severe. The first type of MS is Relapsing-Remitting MS (RRMS) in which there are acute and unpredictable exacerbations (acute attacks, also called flare-ups). Over 80% of patients start off with this type. During this period symptoms get worse and there are periods of full or partial recovery, but sometimes no recovery at all. The attacks may evolve over days or even weeks, and recovery can take weeks, or even months. In between the attacks there is calm, and symptoms do not worsen. Although there are over a dozen medications approved by the U.S. Food and Drug Administration (FDA) for the treatment of relapsing- remitting MS (RRMS), clinical evidence on whether any of them significantly impede or slow the disease progression is conflicting.
The second type is Primary-Progressive MS (PPMS). About 15% of patients have this type. There are no clear relapses or remissions and the progression of the disease is steady. It is the most common form of MS in those who develop the disease after 40 years of age. Currently there are no medications that have been specifically approved by the FDA for the treatment of primary-progressive MS. However, many neurologists prescribe the medications indicated for the relapsing- remitting course in the hope it may slow progression
The third type is Secondary-Progressive MS (SPMS) which starts off as the relapsing-remitting type of multiple sclerosis. Relapses and partial recoveries occur. However, in between the cycles, ever-increasing disability does not go away. Eventually it becomes a progressive disease with no cycles. The progressive stage may start very early on, or years, and even decades later. With few risk-free alternatives available,
neurologists often continue to prescribe the same medications that the patient took during the relapsing- remitting phase beyond the point where there is evidence that the drug is able to do an acceptable job of controlling disease activity (Lublin FD. et al. 2014).
Signs and symptoms of MS vary widely and depend on the amount of nerve damage and which nerves are affected. Two of the most common symptoms are fatigue and difficulty walking. About 80 percent of people with MS report having fatigue. Fatigue that occurs with MS is more than just feeling tired. It can become debilitating, affecting the ability to work, focus on and perform everyday tasks.
Common symptoms of multiple sclerosis are bladder problems, bowel problems, cognitive function, depression, emotional changes, dizziness and vertigo, numbness or weakness, sexual dysfunction, spasticity and muscle spasms, tremor, vision problems and gait changes. The most commonly reported cognitive abnormalities include executive functional challenges with memory, abstraction, attention and word finding.
[Nordqvist, C., Multiple Sclerosis: Causes, Symptoms and Treatments (2016, March 09). https://www.medicalnewstoday.com/articles/37556.php?page=2.]
It isn’t clear why MS develops in some people and not others. A combination of genetics and environmental factors appears to be responsible although that does not explain entire ethnic groups who are at low risk within populations where the disease is prevalent. Many factors may increase the risk of developing multiple sclerosis. Age is a factor since, while MS can occur at any age, it most commonly affects people between the ages of 15 and 60, with many fewer cases seen before the age of 30.
[“How age affects Multiple Sclerosis Symptoms and Progression”.- 2015
https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS. Polliack ML et al 2001) ]
Another factor is gender with women being about twice as likely as men to develop MS. Family history is also a factor since, if parents or siblings have MS, one is at a higher risk of developing the disease. Other factors include certain infections, for example, a variety of viruses have been linked to MS, including Epstein-Barr, the virus that causes infectious mononucleosis, herpes virus 6, herpes simplex virus, influenza, measles, mumps, varicella-zoster virus, cytomegalovirus (CMV), and respiratory syncytial virus (RSV) and Chlamydia pneumoniae, a bacterial infection. There is no evidence that any type of vaccine causes multiple sclerosis.
[Retrieved from Multiple Sclerosis 2012 https://umm.edu/health/medical/reports/articles/multiple-sclerosis.]
Caucasians, particularly those of Northern European descent, are at the highest risk of developing MS. People of Asian, African or Native American descent have the lowest risk. MS is far more common in countries of high latitudes, including Canada, the northern United States, the UK, Scotland, Scandinavia, New Zealand, southeastern Australia, northern Europe, Russia, and northern Asia (Simpson Jr. S et al 2011). Certain autoimmune diseases are a factor since a person has slightly higher risk of developing MS if they have thyroid disease, type 1 diabetes
or inflammatory bowel disease. Smokers who experience an initial event of symptoms that may signal MS are more likely than nonsmokers to develop a second event that confirms relapsing-remitting MS.
[https://www.mayoclinic.org/diseases-conditions/multiple- sclerosis/symptoms-causes/dxc-20131884]
Individuals with a traumatic brain injury are also at an increased risk of developing MS. (Freire MA 2012). A medical history and complete neurologic exam and some lab tests along with MRI imaging are needed to diagnose MS. There are no specific tests for MS. Instead, a diagnosis of multiple sclerosis often relies on ruling out other conditions that might produce similar signs and symptoms, known as a differential diagnosis. Doctors are likely to start with a thorough medical history and examination. Doctors may then recommend blood tests to help rule out other diseases with symptoms similar to MS. Tests to check for specific biomarkers associated with MS are currently being sought but the consensus revision of diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria is the gold standard for diagnosing the disease. (Polman C. et al 2011)
Also recommended is a lumbar puncture (spinal tap), in which a small sample of cerebrospinal fluid (CSF) is removed from the spinal canal for laboratory analysis. This sample can show abnormalities in proteins, products of myelin destruction and antibodies that correlate closely with the clinical activity of MS (Cohen, SR 1980); specifically oligoclonal IgG bands (not found in the serum of the patient) or an elevated IgG index. A spinal tap can also help rule out infections and other conditions with symptoms similar to MS (Polman C. et al 2011).
An MRI may also be recommended, which can reveal areas of MS (lesions) on the brain and spinal cord. A patient may receive an intravenous injection of a contrast material to highlight lesions that indicate the disease is in an active phase. (Polman C, et al. 2011) In most people with relapsing-remitting MS, the diagnosis is fairly straightforward and based on a pattern of symptoms consistent with the disease and confirmed by brain imaging scans, such as MRI. Diagnosing MS can be more difficult in persons with unusual symptoms or a progressive early form of the disease. In these cases, further testing with spinal fluid analysis and additional imaging may be needed.
[Pietrangelo, A. Understanding Multiple Sclerosis. (n.d.) Retrieved from www.healthline.com/health/multiple-sclerosis, (Polman C, et al. 2011)]There is currently no cure for multiple sclerosis. Patients diagnosed with MS have been subject to a decline of functional abilities until death. MS patients live 10-15 years less than average despite available medications. Interventional pharma-based therapies are targeted at symptomatic treatment only and have not been effective for either a reduction of symptoms over time or restoration of function. Historically, the standard treatment for Multiple Sclerosis (MS) and other chronic neurological diseases of a demyelinating nature has been mainly pharmacological, with the intent of returning
neurological function after an attack, inhibiting new attacks and preventing disability. (Rice C. 2014)
Medications for MS have long-term side effects such as muscle aches, fatigue, cardiac issues, allergic reactions and headaches, and additionally some are immunosuppressive. Moreover, because of the degenerative nature of MS, the disease can only be minimally controlled, not cured. Therefore, as these drugs only modify symptoms and do not slow down the rate at which neurons are lost, it is necessary to increase dosages or introduce new medication, such as (beta) 3- inhibitors. These increased dosages by themselves introduce more potential health risks to patients.
Treatments for acute attacks or flares of MS may include oral or intravenous corticosteroids or plasma exchange. Corticosteroids, such as oral prednisone and intravenous methylprednisolone, are prescribed to reduce nerve inflammation. Side effects may include insomnia, increased blood pressure, mood swings, elevated blood sugar, drug-induced psychosis and fluid retention.
Plasma exchange electrophoresis is a technique where the liquid portion of blood (plasma) is removed and separated from blood cells. The blood cells are then mixed with a protein solution (albumin) and put back into the body. Plasma exchange may be used if symptoms are new, severe and haven’t responded to steroids (Tumani H. 2008).
At this time, there are no FDA-approved treatments available for slowing the progression of primary- progressive MS (PPMS) or secondary-progressive MS (SPMS). For relapsing-remitting MS (RRMS), several disease-modifying therapies are available. Much of the immune response associated with MS occurs in the early stages of the disease. It is claimed that aggressive treatment with these medications as early as possible can lower the relapse rate and slow the formation of new lesions. Many of the disease-modifying therapies used to treat MS carry significant health risks. Selecting the right therapy depends on careful consideration of many factors, including duration and severity of disease, effectiveness of previous MS treatments, other health issues, cost, and childbearing status.
Oral pharmacologic treatments are numerous and include beta interferons which are among the most commonly prescribed medications for the treatment of MS. They are injected under the skin or into muscle and can reduce the frequency and severity of relapses, although there is no evidence they change the course or timelines of the disease. Glatiramer acetate (Copaxone (R)) may help block the immune system’s attack on myelin and must be injected beneath the skin. Teriflunomide (Aubagio(R)) is a once-daily medication, which may reduce relapse rate. Natalizumab (Tysabri (R)) is designed to block the movement of potentially damaging immune cells from the bloodstream to the brain and spinal cord. It has been used as a first line treatment for some people with severe MS or as a second line treatment in others. Dimethyl fumarate,
(Tecfidera(R)) has been approved for the treatment of patients with relapsing forms of multiple sclerosis. Alemtuzumab, (Lemtrada(R)) has been approved for the reduction of relapses of MS by targeting a protein on the surface of immune cells and depleting white blood cells. This effect is claimed by the manufacturer to potentially limit nerve damage caused by the white blood cells, but it also increases the risk of infections and autoimmune disorders. (Rice C. 2014) In some cases, treatments have been provided for MS signs and symptoms such as physical therapy and even possibly muscle relaxants such as baclofen (Lioresal (R)) and tizanidine (Zanaflex(R)). In other cases medications have been administered to reduce fatigue. Further, medications have been prescribed for depression, pain, sexual dysfunction, and bladder or bowel control problems that are associated with MS. Activities such as exercise, meditation, yoga, massage, eating a healthier diet, acupuncture and relaxation techniques may help boost overall mental and physical well-being, but there are few studies to back up their use in managing symptoms of MS.
[https://www.mayoclinic.org/diseases-conditions/multiple- sclerosis/diagnosis-treatment/treatment/txc-20131903.]
Other potential treatments include minimally invasive dilatation of the jugular veins, put forward in a hypothesis by Paolo Zamboni in 2009. Clinical trials have not proven the efficacy of vein dilatation because of the jugular veins’ tendency to restenose after a short time (average <90 days). Furthermore, much anecdotal evidence of damage intraluminally has been reported by MS patients as a result of neck vein catheterization and dilatation. Accordingly, the problems with this technique render this process ineffective. (Tsivgoulis G, 2015.)
Stem cell therapy for the treatment of MS has been attempted wherein most or all clinical human studies using stem cells for the treatment of MS employ the administration of a particular amount of stem cells into the hand or the arm of the patient. The dorsal metacarpal veins in the hand and the cephalic and basilic veins in the forearm are the veins most often referred to or used for initiating intravenous (IV) therapy when infusing stem cells. The methodologic problem with using such a peripheral vein, including the IJV to infuse stem cells is that many of the infused stem cells will become sequestered in the lungs and to a lesser extent the liver and spleen and not reach the brain of the MS patient (Fischer, UM, 2009, Furlani et al 2009).
Stem cell therapy has further been used in attempts to treat other diseases and conditions, for example, they have been used to treat myocardial and spinal injury as disclosed in US Application 2005/0015048, US application 2013/0338637 and US application 2012/0156230.
Despite all such advances and attempts, a need remains for a more effective treatment of a patient suffering from vascular-related degenerative neurological disease(s), particularly multiple sclerosis. The current invention is directed to this and other needs.
Results: Study Subject Walk Test
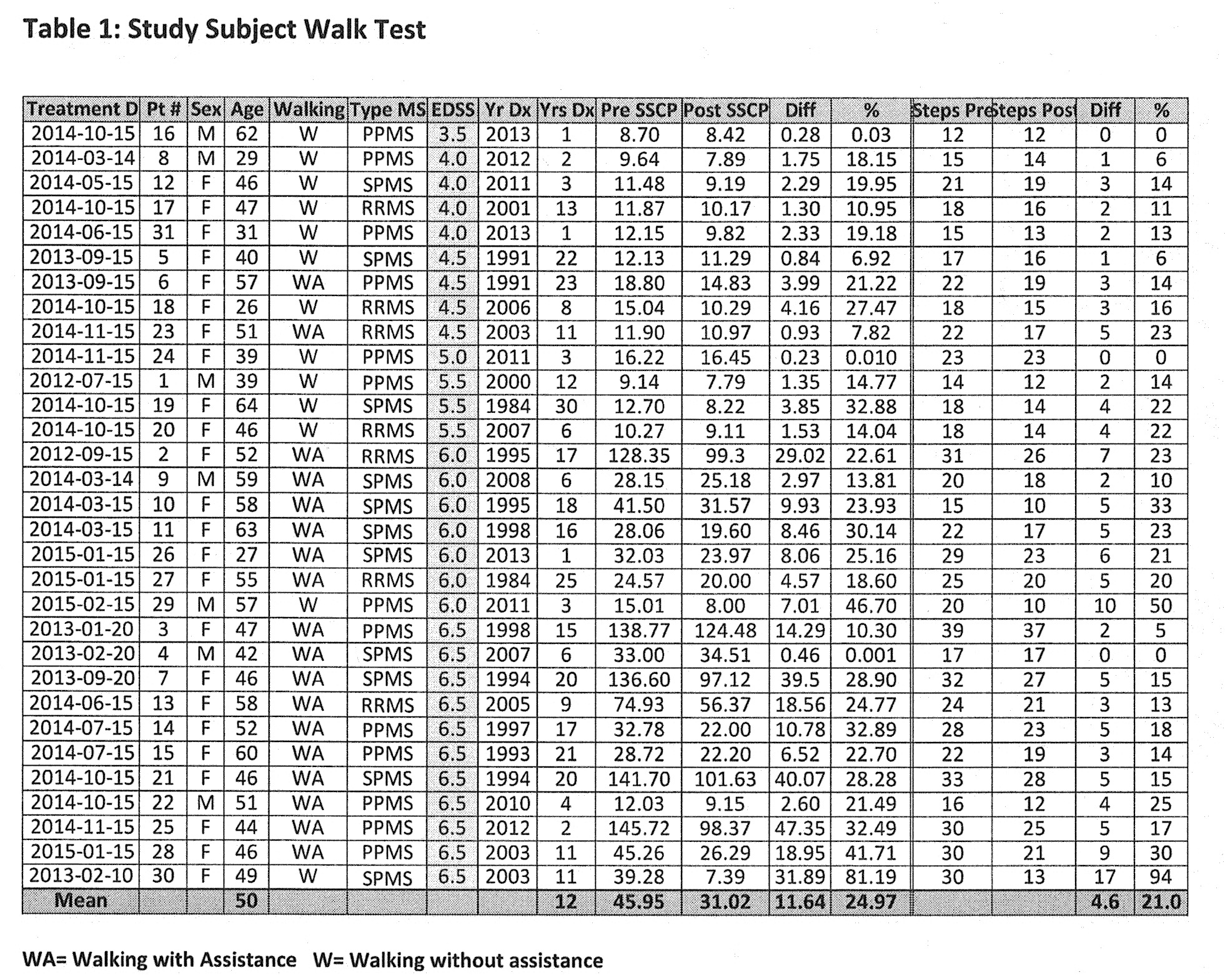
The differences between pre and post-therapy walk times were analyzed. Despite wide ranging abilities, 27 of 31 subjects demonstrated changes of statistical significance. With an average of about 46 seconds to complete the pre-therapy walk test, the mean improvement post-therapy is 22.55%, adjusted from 24.97% (SD 15.7%, 95% CI), significant to p < 0.05. No adverse effects were observed. As can be seen from the results shown in this table, the method of the present invention significantly improved the time taken for a patient to complete the Timed 25 Foot Walk post-therapy regardless of degree of disability.