SARS-CoV2 first line of defense
Treatment Trial
To evaluate Safety & Efficacy of established medicine in controlling COVID-19 SARS-CoV2 infection in a home-quarantine environment
Surjo Banerjee*
Sponsor: Somata Genesis, Inc., Idaho, USA
Trial site: Somata Genesis Biotech (P.) Ltd., Pune, India
*Correspondence: surjo@somatagenesis.com
Preprint doi: 10.13140/RG.2.2.24554.67526/3. The copyright holder for this preprint is the author/funder. This research available under a CC-BY-ND 4.0 International license.
1 of 15
Abstract
This treatment protocol was designed to inhibit SARS-CoV2 infection within its incubation phase; suppress aerosolization of replicated virions; boost immune response against infection; and prevent pro-inflammatory cytokine release. Achievement of these objectives would limit replication and spread of SARS-CoV2 to other humans sharing quarantine space and incapacitate the infection from causing critical pro-inflammatory damage in a home-quarantined patient.
Keywords
COVID-19, SARS-CoV2, 2019-nCoV, Coronavirus, pandemic, quarantine, public health
Introduction
Due to the mounting intensity of COVID- 19 infections worldwide, more countries are forced to impose massive lockdowns to contain clusters within their territory. Such lockdowns are not aimed at minimizing infections but containing further spread. Confining civilians within their homes without adequate training on aseptic practices and continued exchange of external supplies, maximizes the risk of infection for all members sharing quarantine space. SARS-CoV2 can sustain their integrity longer in consistently cool (20-25°C), humid confines of a residential building. The first infected member can synthesize and aerosolize millions of virions within days to be gradually picked up by remaining members within one quarantine space. A SARS-CoV2 infected human may appear asymptomatic for up to a week while aerosolizing virions. During that time, millions of virions would have contaminated the shared quarantine space. The current measured R0 (R naught)
for SARS-CoV2 is ~2.2 to 2.4 which is the average number of individuals, one patient can infect. Lockdowns can significantly increase R0 due to proximity between individuals confined to their homes.
Most countries are not equipped to manage the Covid19 pandemic at critical stages of the infection. Therefore, current unpredictable circumstances may lead to inaccessibility to centralized healthcare in regions severely affected. Through this study, the treatment efficacy of locally available pharmaceuticals and nutrition is evaluated to prevent SARS-CoV2 infection from spreading amongst quarantined members and reaching critical stages, thereby reducing the burden on healthcare institutions.
Materials and Methods
Inclusion criteria: SARS-CoV2 infected patient (M/F) within age-group: 20-40 years; exhibiting moderate to severe (non-critical)
2 of 15
symptoms of SARS-CoV2 infection; treatable with oral medication.
Exclusion criteria: SARS-CoV2 patient with digestive disorder, severe hypoxia (tachypneic or dyspneic symptoms) or with underlying respiratory disease, diabetic & cardiovascular conditions, patient taking immunosuppressants. Paucisymptomatic but slightly hypoxic patients should always consider oxygen therapy as the first therapeutic approach.[1]
Baseline: Somata Genesis studied the effects of established medicine on a 28- year old female patient at day 3 of exhibiting classic and most common SARS-CoV2 symptoms at moderate to severe intensities.
At the time of inclusion, the subject experienced hemoptysis, indicating the onset of lower respiratory tract infection. Signs of sepsis indicated by dyspnea and hypoxemia, reduced urine output, tachycardia and depressed mental status. Symptomatic diagnosis at the time of inclusion:
| Fever | Mild (~99°F) |
| Coughing + sputum/sneezing | Severe |
| Pharyngitis | Severe |
| Laryngitis | Severe |
| Dyspnea | Mild |
| Hemoptysis | Mild |
| Nausea | Mild |
| Muscular/joint pain | Severe |
| Pneumonia | Mild |
Comparing the above baseline symptoms to progression data released by the Singapore Health Ministry, suggests that the subject may have been at approx. day 10 of SARS-CoV2 infection at the time of inclusion. The subject reported noticeable symptoms on the 7th day (morning) since transiting through two international airports. Subject transited through the same airports again on the 10th day since her first transit. Treatment began on the 11th day (morning) since her first airport transits. Therefore, it is further validated that the infection may take up to 7 days to reflect noticeable symptoms in healthy adults. The subject reported feeling nauseous and dehydrated; with mild bladder incontinence, low and slightly painful urine output and constipation. Digestion appeared normal; favoring oral administration of medicines and nutrition; meeting requirements of treatment in home-quarantine. Antiemetic medication was kept on standby.
1. Inhibit infection within incubation phase
A. Povidone-Iodine
Method: For each session, 30 ml of solution was freshly prepared using 15 ml Povidone-Iodine germicide 2% mixed with 15 ml luke-warm water (~40°C) to cleanse the oral entry; oropharynx. Subject gargled freshly prepared solutions, every 3 hours for the first 12 hours. The frequency of gargles was gradually reduced to one gargle per day by treatment day 5. Each session consumed 30 ml; 2 gargles in each session using a 15 ml
3 of 15
solution each time; each gargle took 2-3 minutes.
Observation: Subject experienced significant reduction in throat irritability with each session. Noticeable reduction in coughing and sneezing was observed. Sputum was ejected immediately after each oral rinse with traces of blood (hemoptysis) indicating acute bronchitis or pneumonia. The immediate relief from irritation and expectorated sputum indicated that the actions of the irritating agent were subdued effectively using Povidone-Iodine.
Mechanism of Action: Inhibiting the entry and replications of the virus within the upper respiratory tract and destroying newly formed virions was planned using Povidone-Iodine, a readily available germicidal gargle / oral rinse. SARS- CoV2 is effectively inactivated by lipid solvents including ether (75%), ethanol, chlorine-containing disinfectants, peroxyacetic acid and chloroform except for chlorhexidine. [1] Chlorhexidine Gluconate, which is another readily available oral rinse known to damage some fragile virions is therefore deemed ineffective on SARS-CoV2. At the time of this consideration however, no data was available to compare the effectiveness of Povidone-Iodine over Chlorhexidine Gluconate. Povidone-Iodine was particularly chosen for its broad- spectrum damaging capabilities on most pathogens within 15 seconds of administration, thereby increasing the chances of destroying a large number of
newly synthesized virions it came in contact with.
Side-Effects: Povidone-Iodine contains Iodine which is primarily taken in through diet, with the recommended amount at 150 μg per day in adults who are not pregnant or lactating. Although excess iodine exposure generally does not result in any apparent clinical consequences, thyroid dysfunction can occur in vulnerable patients with specific risk factors, including those with pre-existing thyroid disease, the elderly, fetuses and neonates. As iodine-induced hypothyroidism or hyperthyroidism might be either subclinical or overt, excess iodine exposure should be suspected if the etiology of thyroid dysfunction is not discernible. [2] As a result, some adult females may experience menstrual irregularities such as delay of up to 10 days in their next mensuration.
2. Suppress aerosolization of replicated virions
B. Dextromethorphan Hydrobromide & Chlorpheniramine Maleate syrup
Method: Coughing nasal/throat irritation was controlled by orally administering 10 ml of Dextromethorphan Hydrobromide & Chlorpheniramine Maleate syrup after every gargle session and as required.
Observation: Immediate halt in coughing, sneezing, relief from throat irritation was noticed, thereby preventing aerosolization of virions.
4 of 15
3. Boost immune response against infection
C. Multivitamin nutraceutical syrup
Method: A 10 ml dose of multivitamin nutraceutical syrup was orally administered in fasted state, twice a day till treatment day 4. The dose was cut down to half by administering a single dose of 10ml in fasted state on days 5, 6 and 7.
Active ingredients: D-Panthenol, Dl- Methionine, Elemental Iron, Folic Acid, Inositol, Iodine, Lycopene, Manganese Sulphate, Methylcobalamin, Molybdenum, Niacinamide, T aurine, Vitamin A, Vitamin B1, Vitamin B2, Vitamin B6, Vitamin C, Vitamin D3, Vitamin E, and Zinc Sulphate.
Mechanism of Action: A Vitamin C rich (20 mg / 10 ml) multivitamin nutraceutical syrup was chosen. Vitamin C contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune systems. [3] Deficiency of Vitamin C in a patient can result in impaired immunity and higher susceptibility to infections.
D. Garlic (Allium sativum)
Method: One raw clove of garlic (Allium sativum) peeled (~10 gm) was orally administered every day in fasted state for the first 3 days of treatment.
Active ingredients: S-allyl-L-cysteine (SAC), S-allyl-L-cysteine sulfoxide (alliin), S-allyl mercaptocysteine (SAMC), γ-glutamyl cysteine derivatives, Diallyl disulfide (DADS), Sulfide family compounds, (E–Z)-ajoene
compounds and Dithiines.
Mechanism of Action: Compounds in Allium sativum have a broad spectrum of beneficial effects against microbial infections. These compounds have cardioprotective, anticarcinogenic, anti- inflammatory and immunomodulatory effects. Immunoregulatory mechanism: Modulating cytokine secretion, Phagocytosis and cell activation, Activation of humoral immune response and synthesis of Immunoglobulin A (B- cell stimulation), Antiallergic response, Mitogenic stimulation. [4]
E. Radish (Raphanus sativus)
Method: Subject received a daily serving of radish/daikon 500 gm (Raphanus sativus variety Longipinnatus) in raw state to supplement 110 mg~ of additional Vitamin C in the body.
Active ingredients: water-soluble vitamins C (36%), B1, B2, B3, B5, B6 and B9; minerals calcium (2%), iron (2%), magnesium (4%), manganese zinc, potassium, and phosphorous. [5]
Known primary bioactive compounds include glucosinolates: glucoraphanin, 4- hydroxyglucobrassicin, glucoerucin, glucoraphasatin, glucobrassicin, 4- methioxyglucobrassicin, and neoglucobrassicin and isothiocyanates: sulforaphene, sulforaphane, and indole- 3-carbinol. [6, 7, 8] Among other things, myrosinase, glucosinolate, and isothiocyanate [9] are associated with
5 of 15
anticancer activities. 4-Methylsulfinyl-3- butenyl isothiocyanate present in Raphanus sativus induces apoptosis in human lung cancer cells [10, 11].
*Vitamin C
*Total intake per day:
• 110 mg (R. sativus) + 40 mg (multivitamin syrup) = 150 mg (Day 1,2,3,4)
• 110 mg (R. sativus) + 20 mg (multivitamin syrup) = 130 mg (Day 5,6,7)
Mechanism of Action: Vitamin C supports the epithelial barrier function against pathogens. Prophylactic prevention of the infection requires dietary vitamin C intakes that provide at least adequate, if not saturating plasma levels (i.e., 100- 200 mg/day), which optimize cell and tissue levels. Treatment of established infections requires significantly higher (gram) doses of the vitamin to compensate for the increased inflammatory response and metabolic demand. Therefore R. sativus was included as a dietary supplement along with the multivitamin syrup. Vitamin C accumulates in phagocytic cells, such as neutrophils, and can enhance chemotaxis, phagocytosis, generation of reactive oxygen species, and ultimately microbial killing. It is also needed for apoptosis and clearance of the spent neutrophils from sites of infection by macrophages, thereby decreasing necrosis/NET osis and potential tissue damage. Vitamin C has been shown to enhance differentiation and proliferation
of B- and T-cells, likely due to its gene-regulating effects. It is a potent antioxidant and a cofactor for a family of biosynthetic and gene regulatory enzymes. Vitamin C has a pleiotropic ability to donate electrons. It promotes the oxidant scavenging activity of the skin, thereby potentially protecting against environmental oxidative stress. [3]
4. Preventing pro-inflammatory cytokine release
F. Amoxicillin/clavulanate potassium
Method: 625 mg of amoxicillin/clavulanate potassium was orally administered in fed state, one hour post the first dose of 10 ml multivitamin syrup. Another dose was administered in fed state on treatment day 2.
Mechanism of Action: Amoxicillin / clavulanate potassium belongs to the penicillin group of antibiotics used to treat a broad spectrum of infections caused by bacteria, such as lower respiratory tract Infections, sinusitis, pneumonia, bronchitis, Otitis Media, ear infections, urinary tract infections, and infections of the skin. [12]
This antibiotic was administered to eliminate secondary respiratory infections caused by opportunistic pathogens. Amoxicillin/clavulanate potassium has recorded no adverse effects on immunity in adult humans and can bind directly to performed MHC- peptide complexes and need not necessarily involve the processing of haptenated, self-carrier proteins before recognition of the conjugate by amoxicillin-
6 of 15
specific T cells. [13] This is particularly beneficial against SARS- CoV2 aborting T cell responses by inducing pro-inflammatory cytokines into macrophages and dendritic cells. [14, 15] Amoxicillin/clavulanate potassium’s action clandestinely increases T cell activity without getting in the way of SARS-CoV2 [16], thereby avoiding its virions from releasing pro-inflammatory cytokines.
G. Ginger (Zingiber officinale Rosc.)
Method: The subject chewed raw ginger (Z. officinale); ~4 gm per serving per day for the first 3 days of treatment. Z. officinale has natural anti-inflammatory properties known to reduce soreness in the throat, muscle pain, septic shock and inhibit the effects of pro-inflammatory cytokines.
Mechanism of Action: [6]-gingerol is believed to be the most abundant bioactive component of ginger root amongst 115 identified constituents [17]. Gingerol, shogaol, and other structurally- related substances in ginger [18]:
-
Inhibit prostaglandin and leukotriene biosynthesis through suppression of 5-lipoxygenase or prostaglandin synthetase.
-
Inhibit the synthesis of pro- inflammatory cytokines such as IL-1, TNF-α, and IL-8.
-
In macrophages, shogaol can down-regulate inflammatory iNOS and COX-2 gene expression.
-
Rhizome hexane fraction extract of Z. officinale inhibits excessive production of NO, PGE (2), TNF- alpha, and IL-1beta.
-
Gingerols can inhibit LPS-induced COX-2 expression while shogaol containing extracts have no effect on COX-2 expression.
-
Potent compounds in ginger rhizome inhibit allergic reactions.
-
Compounds present in ginger are capable of inhibiting PGE (2) production.
-
Ginger extracts have hypoalgesic effects (decreased sensitivity to pain). Daily consumption of raw or heat-treated ginger can result in moderate-to-large reductions in muscle pain.
Ginger brings about anti-inflammatory effects through the modulation of calcium levels mediated through transient receptor potential vanilloid subtype 1 (TRPV1), which is a heat-and pain- sensitive receptor that interacts with [6]- gingerol.
Ginger constituents can reduce elevated expression of NFκB and TNF-α in rats with liver cancer. The activation of NF-κB is linked to a variety of inflammatory diseases, including cancer, multiple sclerosis, Alzheimer’s disease, atherosclerosis, myocardial infarction, diabetes, allergy, asthma, arthritis, Crohn’s disease, osteoporosis, psoriasis, septic shock, and AIDS.
7 of 15
Standby medication
Additional medication was kept on standby to relieve the subject of any discomfort that might arise from the infection or any side-effects from the doses administered which might prevent adequate rest/sleep during recovery.
Standby medication
| Indication | Medication |
| Cardialgia (acid indigestion) | Aluminum Hydroxide + Magnesium Hydroxide + Dimethicone antacid liquid |
| Mild fever | Nimesulide 100mg |
| High fever + Fatigue | Nimesulide 100mg + Paracetamol 325mg |
| Mild fever + phlegm | Paracetamol 500mg + Phenylephrine Hydrochloride 10mg + Caffeine Anhydrous – 32 mg |
| Nausea | Promethazine 25mg |
Precautions
Antipyretics Nimesulide and Paracetamol are not very effective nor recommended in the treatment of viral fever. Some of these, present the risk of critical side-effects. To allow adequate rest, a combination of Nimesulide and Paracetamol was effective in controlling fever and discomfort in the subject to allow adequate rest. Systemic corticosteroids for the treatment of viral pneumonia or acute respiratory distress syndrome (ARDS) are not recommended. [1]
SARS-CoV2 Symptomatic Intensity Scale [S2-SIS]
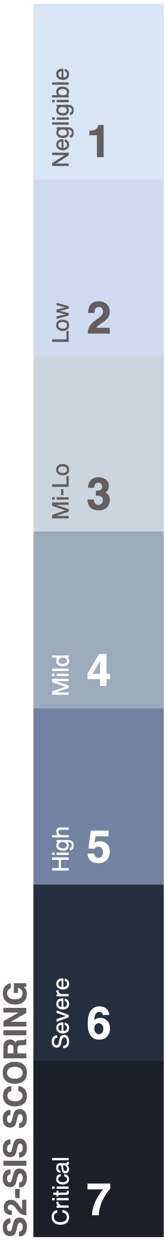 |
A scaling standard was established to compare retrospective data with post-therapeutic recovery. Dosing and subjective assessment of safety and efficacy were carried out by this standard. All symptoms were quantified based on clinical evaluation of current intensity.Symptomatic intensities were individually measured on a daily basis and total intensity [S2-SIS] was calculated by adding up all individual symptom measurements. |
8 of 15
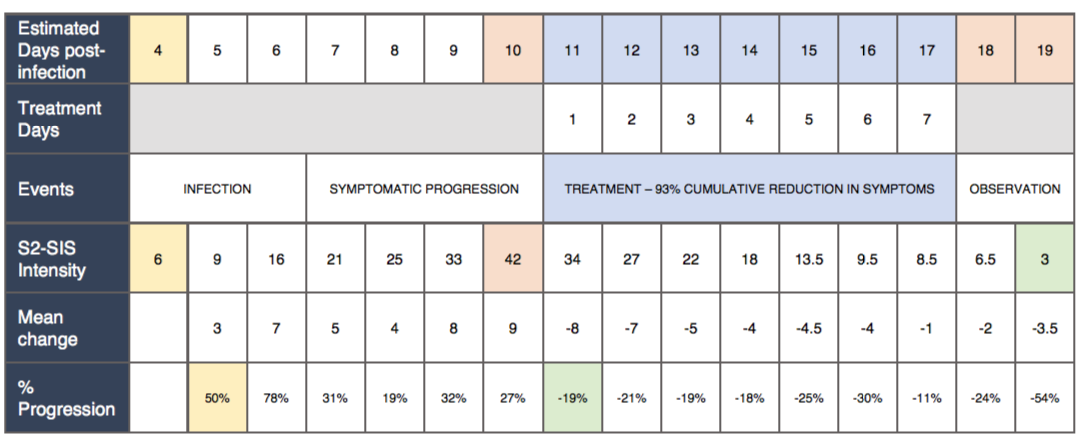
Total Dosage: 1-week Symptomatic Treatment and Rate of Response
| Treatment goal | Pharmaceuticals / Nutrition | DAY1 | DAY2 | DAY3 | DAY4 | DAY5 | DAY6 | DAY7 | ||||||||||||||||||||||||
| Reduction in Symptoms | -19% | -21% | -19% | -18% | -25% | -30% | -11% | |||||||||||||||||||||||||
| Inhibit infection | Povidone-Iodine germicide 2% gargle 15 ml +15 ml H2O (40°C) | 15ml x 4 | 15ml x 4 | 15ml x 3 | 15ml x 2 | 15ml x 1 | 15ml x 1 | 15ml x 1 | ||||||||||||||||||||||||
| Suppress Aerosolization | Dextromethorphan Hydrobromide & Chlorpheniramine Maleate syrup | 10ml x 3 | 10ml x 3 | 10ml x 3 | 10ml x 3 | 10ml x 1 | 10ml x 1 | 10ml x 1 | ||||||||||||||||||||||||
| Boost immunity |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
| Preventing cytokine storm syndrome |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
| Fever/cold relief (Standby medication) |
|
|
|
|
|
|
|
|
Post-therapeutic Observations – Treatment Day 1 to Day 10 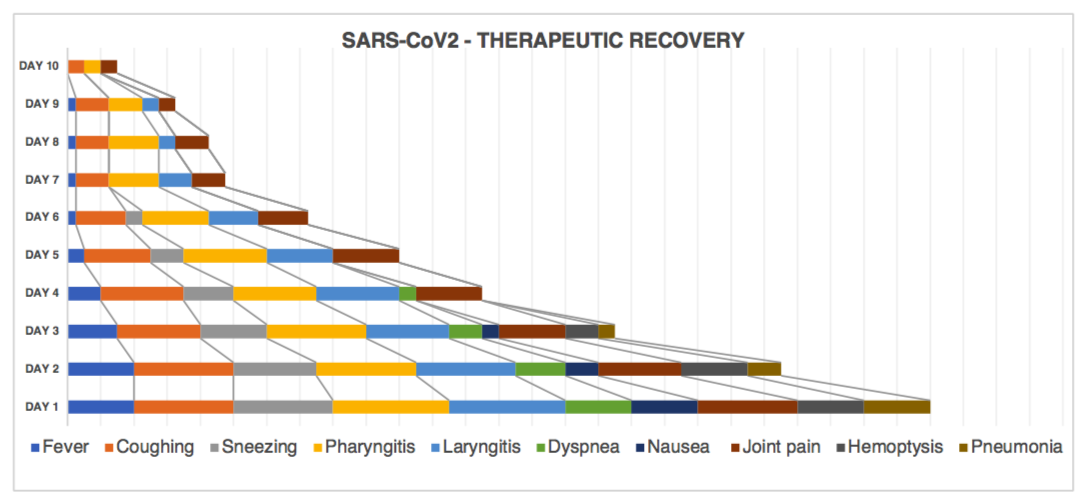
9 of 15
Results – S2-SIS Evaluation

| Treatment | Day1 | Day2 | Day3 | DAY4 | DAY5 | DAY6 | DAY7 | DAY8 | DAY9 | DAY10 |
| Fever | ~99°F | ~99°F | ~98°F | ~97°F | ~96.8°F | ~96°F | ~96°F | ~96°F | ~96°F | ~95.9°F |
| Coughing | Severe | Severe | High | High | Mild | Mi-Lo | Low | Low | Low | Negligible |
| Sneezing | Severe | High | Mild | Mi-Lo | Low | – | – | – | – | – |
| Pharyngitis | Critical | Severe | Severe | High | High | Mild | Mi-Lo | Mi-Lo | Low | Negligible |
| Laryngitis | Critical | Sever | High | High | Mild | Mi-Lo | Low | Negligible | Negligible | – |
| Dyspnea | Mild | Mi-Lo | Low | Negligible | – | – | – | – | – | – |
| Nausea | Mild | Low | Negligible | – | – | – | – | – | – | – |
| Joint Pain | Sever | High | Mild | Mild | Mild | Mi-Lo | Low | Low | Negligible | Negligible |
| Hemoptysis | Mild | Mild | Low | – | – | – | – | – | – | – |
| Pneumonia | Mild | Low | Negligible | – | – | – | – | – | – | – |
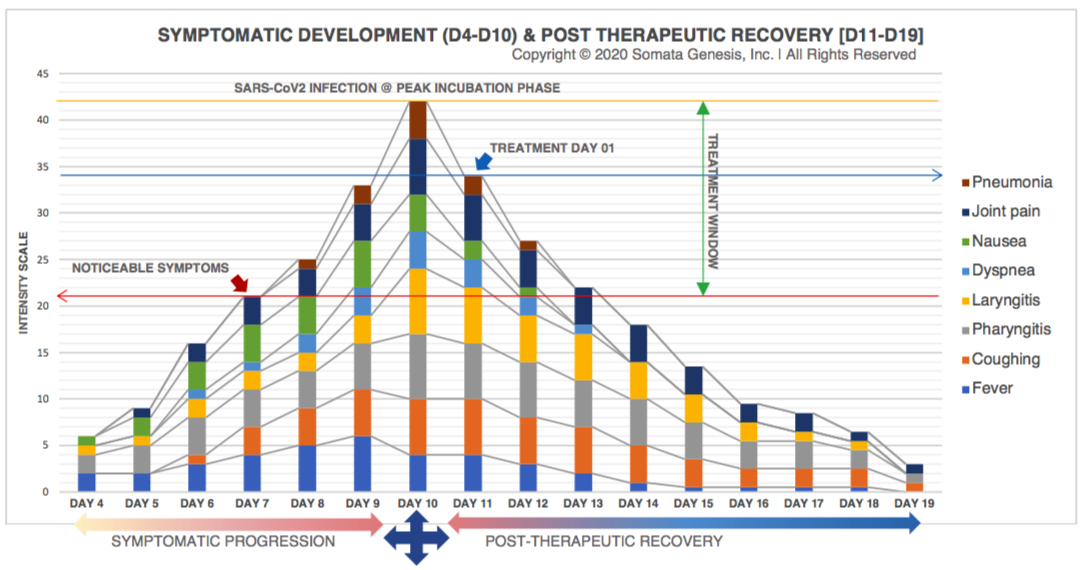
10 of 15
-
Total Two symptomatic parameters; sneezing and hemoptysis were left out from the symptomatic intensity scale [S2-SIS totals] due to the unavailability of retrospective data for comparison.
-
The subject has remained under 24×7 observation for 26 days since the beginning of her treatment.
-
The subject showed no signs of sleep disorder; completed the daily sleep-cycle of eight hours without any need for additional medication to do so.
-
All symptoms were down to zero by treatment day 10 and have not returned
-
Subject experienced ~8 days of delay in her menses; supposedly due to the temporary overproduction of TH from partial ingestion of Povidone-Iodine gargle solutions and Iodine supplementation through multivitamin intake.
-
The subject continues to remain under 24×7 observation.
-
No adverse events were reported during or since her treatment
Personnel assisting subject during the treatment and observation were limited to two individuals who were trained to follow aseptic techniques in a home quarantine setup. Both individuals have been observed for signs of infection for a period of 26 days; they have not contracted any infection whatsoever.
Discussion
With currently available medicines and nutrition, it is possible to control SARS-CoV2 infections within the incubation phase before the following are indicated: (1) Septic shock/cytokine storm syndrome; (2) Proteinaceous/sputum exudates from lungs, edema, viral pneumonia. The studied symptomatic treatment protocol has successfully controlled a SARS-CoV2 infection at the incubation phase in a 28-year old female subject, by conventional oropharynx cleansing; suppressing virion aerosolization; nutritional enhancements to the immune system; anti-inflammatory supplementation and antibiotics to control secondary infections. This is achieved through oral administration of readily available medications and nutritional foods over a period of 7 days. A cumulative symptomatic reduction score of 93% has been achieved within this treatment period.
Acknowledgments
For over a decade, Somata Genesis has been studying the pathogenesis of Coronavirus and its link to autoimmune disorders for which the company develops treatments. We wish to thank our research participant and all the team members who made this study possible. Somata Genesis also thanks the entire global scientific community which is achieving concurrent breakthroughs in understanding and controlling the spread of SARS-CoV2.
11 of 15
Other therapies under study to treat severe cases (out of scope of this research)
Oxygen therapy, intubation and protective mechanical ventilation, non-invasive ventilation, nitric oxide gas inhalation therapy have all shown promise and minimized fatalities.
Some antivirals showing promise (out of scope of this research)
| Medication | Dosage | Interval |
| Lopinavir/Ritonavir | 400/100 mg | every 12 hours |
| Chloroquine | 500 mg | every 12 hours |
| Hydroxychloroquine | 200 mg | every 12 hours |
| Alpha-interferon | 200 mg | every 12 hours |
Data is still insufficient to fully understand all mechanisms of transmission, incubation time, clinical course, and the duration of infectivity of SARS-CoV2. Somata Genesis is actively researching biologics to counter the post-immuno-pathogenic conditions caused by the Coronavirus. As available, it shall gradually make its research public to support the collective effort of healthcare institutions worldwide.
